
This is because we know that the substance has zero entropy as a perfect crystal at 0 K there is no comparable zero for enthalpy. The reason is that the entropies listed are absolute, rather than relative to some arbitrary standard like enthalpy. Note that there are values listed for elements, unlike DH fº values for elements. The Thermodynamics Table lists the entropies of some substances at 25 ✬. Entropy is a measure of molecular disorder or randomness of a system, and the second law states that entropy can be created but it cannot be destroyed. Continue this process until you reach the temperature for which you want to know the entropy of a substance (25 ✬ is a common temperature for reporting the entropy of a substance). Then you can use equation (1) to calculate the entropy changes. Even though equation (1) only works when the temperature is constant, it is approximately correct when the temperature change is small. Now start introducing small amounts of heat and measuring the temperature change.

Since there is no disorder in this state, the entropy can be defined as zero. Imagine cooling the substance to absolute zero and forming a perfect crystal (no holes, all the atoms in their exact place in the crystal lattice). The absolute entropy of any substance can be calculated using equation (1) in the following way. At absolute 0 (0 K), all atomic motion ceases and the disorder in a substance is zero. On this scale, zero is the theoretically lowest possible temperature that any substance can reach.

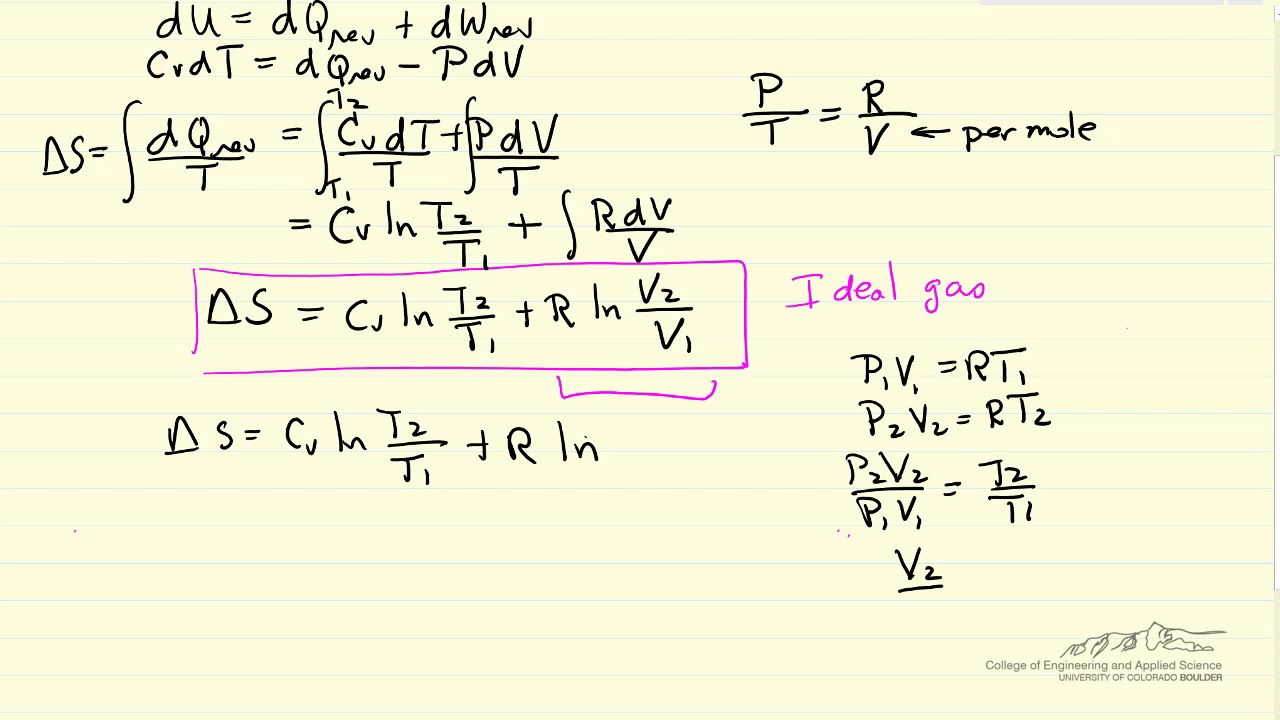
Here Q is the heat transfer necessary to melt 1.00 kg of ice and is given by Q mLf, where m is the mass and Lf is the latent heat of fusion. The temperature in this equation must be measured on the absolute, or Kelvin temperature scale. The change in entropy is defined as: SQT S Q T. Using this equation it is possible to measure entropy changes using a calorimeter. Where S represents entropy, DS represents the change in entropy, q represents heat transfer, and T is the temperature. When entropy increases, a certain amount of energy becomes permanently unavailable to do work.One useful way of measuring entropy is by the following equation:

Entropy is associated with the unavailability of energy to do work. In the second case, entropy is greater and less work is produced. The same heat transfer into two perfect engines produces different work outputs, because the entropy change differs in the two cases. There is 933 J less work from the same heat transfer in the second process. We noted that for a Carnot cycle, and hence for any reversible processes, We can see how entropy is defined by recalling our discussion of the Carnot engine. That unavailable energy is of interest in thermodynamics, because the field of thermodynamics arose from efforts to convert heat to work. Although all forms of energy are interconvertible, and all can be used to do work, it is not always possible, even in principle, to convert the entire available energy into work. Entropy is a measure of how much energy is not available to do work. Recall that the simple definition of energy is the ability to do work. Making Connections: Entropy, Energy, and Work


 0 kommentar(er)
0 kommentar(er)
